UNMATCHED CLINICAL EVIDENCE
The Melody valve is the longest studied TPV, with the largest body of clinical evidence. Accumulated data have consistently demonstrated excellent clinical results, including high rates of freedom from surgical reoperation, confirming the Melody TPV safely and effectively delays the need for surgical conduit exchange.
US IDE Study
Prospective, non-randomized investigational study conducted at 5 centers in the United States. One hundred fifty (150) subjects implanted between January 2007 and January 2010; patients will be followed for 10 years. Data presented are interim results current through June 2016.
US Post Approval Study (PAS)
Prospective, non-randomized study conducted at 10 centers in the United States. One hundred (100) subjects implanted between July 2010 and July 2012; patients will be followed for 5 years. Data presented are interim results through June 2016.
European and Canadian Post-Market Surveillance Study (PMSS)
Prospective, non-randomized study conducted at 7 centers in Europe and Canada. Sixty three (63) subjects implanted between October 2007 and April 2009; patients were followed for 5 years. Data presented are final results as of August 2016.
| Study | # of Centers | # of Patients | First Implant | Last Implant | Mean Length of Follow-up |
|---|---|---|---|---|---|
| US IDE | 5 | 150 | 2007 | 2010 | 6.1 ± 1.7 years |
| US PAS | 10 | 100 | 2010 | 2012 | 3.8 ± 1.2 years |
| EU/CA PMSS | 7 | 63 | 2007 | 2009 | 4.7 ± 1.1 years |
US Investigational Device Exemption Study (IDE) | US Post Approval Study (PAS) | EU/CA Post-Market Surveillance Study (PMSS)
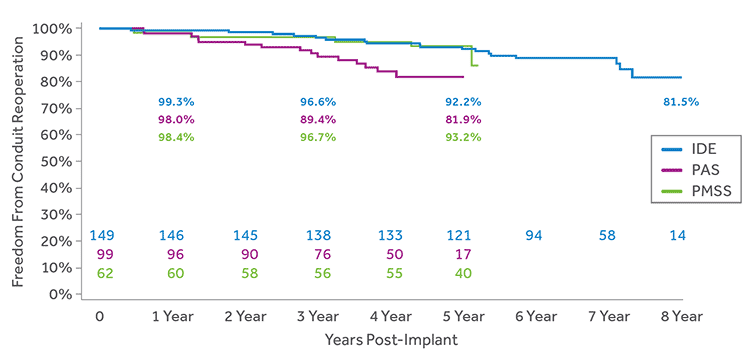
DELAYS PATIENT’S NEXT SURGICAL INTERVENTION
Low rates of surgical conduit reoperation out to 8 years.
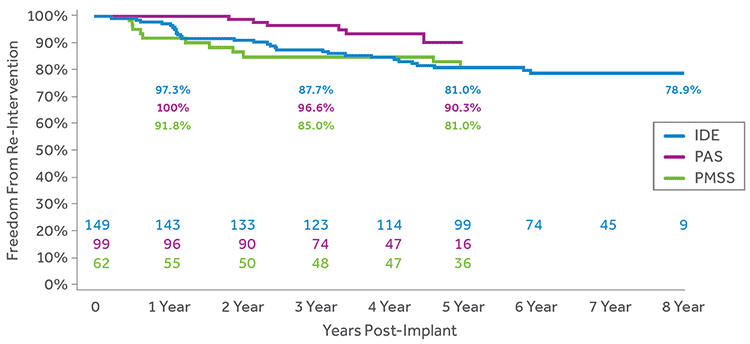
FREEDOM FROM CATHETER RE-INTERVENTION
Freedom from catheter-based re-intervention on the TPV was greater than 78% out to 8 years.
LOW RVOT GRADIENTS
Following Melody TPV implant the mean RVOT gradients decreased and remained consistent throughout follow-up in all 3 studies.
| Mean RVOT Gradient By Time Interval |
Baseline | 1 Year | 3 Year | 5 Year | 7 Year |
|---|---|---|---|---|---|
| US IDE (N=149) |
32.1 ± 13.9 | 18.7 ± 9.1 | 17.6 ± 7.9 | 17.5 ± 8.4 | 17.9 ± 9.8 |
| US PAS (N=99) |
33.4 ± 14.1 | 15.1 ± 7.1 | 16.7 ± 10.8 | 14.4 ± 12.6 | -- |
| EU/CA PMSS (N=62) |
37.7 ± 12.1 | 17.9 ± 9.2 | 17.3 ± 8.4 | 17.3 ± 9.7 | -- |
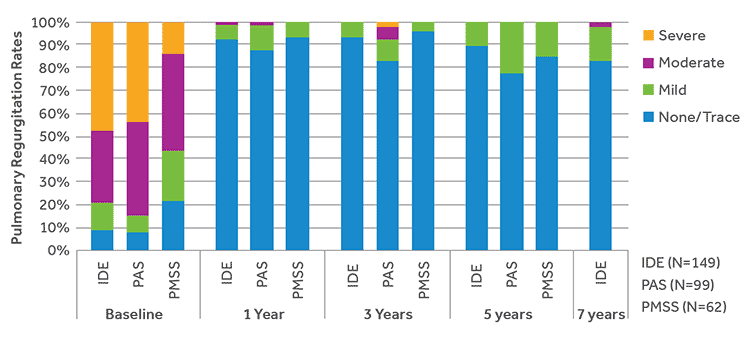
MINIMAL REGURGITATION
The majority of subjects in all 3 studies had a moderate or severe pulmonary regurgitation at baseline. Throughout follow-up, the majority of subjects had no more than trace pulmonary regurgitation.
PROCEDURAL SUCCESS AND STRONG SAFETY PROFILE
The safety profile of the Melody TPV has remained unchanged through the longer-term follow-up data and broader implanter base in the Medtronic studies, demonstrated by the low rates of procedural and device-related serious adverse events.
HIGH RATES OF PROCEDURAL SUCCESS
Consistently high rates of successful valve implantation including strong hemodynamics and low incidence of procedural adverse events.
Procedural success is a composite outcome defined as:
- Melody TPV was successfully delivered to the intended location
- RV-PA peak-to-peak gradient (measured in the catheterization lab) less than 35 mmHg post implant
- Less than mild pulmonary regurgitation
- Free of explant at 24 hours post implant
| Study | Procedural Success |
|---|---|
| US IDE (N=149) | 94.7% |
| US PAS (N=99) | 92.1% |
| EU/CA PMSS (N=62) | 88.7% |
LOW RATES OF DEVICE-RELATED SERIOUS ADVERSE EVENTS
The safety profile of the Melody valve remains out to 7 years as evidence by low rates of serious device-related adverse events across all studies.
| Event | US IDE | US PAS | EU/CA PMSS |
|---|---|---|---|
| Freedom from event at 7 years (CI) (N=149) | Freedom from event at 5 years (CI) (N=99) | Freedom from event at 5 years (CI) (N=62) | |
| Stent Fracture: Major | 83.6% (75.7%, 89.2%) |
91.0% (81.3%, 95.8%) |
91.6% (81.1%, 96.4%) |
| Valve Dysfunction: Stenosis | 79.3% (70.8%, 85.7%) |
86.1% (76.1%, 92.1%) |
82.3% (69.5%, 90.1%) |
| Valve Dysfunction: Regurgitation | 99.3% (95.4%, 99.9%) |
88.7% (75.8%, 94.9%) |
98.3% (89.4%, 99.7%) |
| Prosthetic Valve Endocarditis | 89.2% (79.7%, 94.4%) |
84.9% (73.9%, 91.5%) |
93.2% (82.6%, 97.4%) |
| Embolization of the TPV | 100.0% (NA) |
100.0% (NA) |
100.0% (NA) |
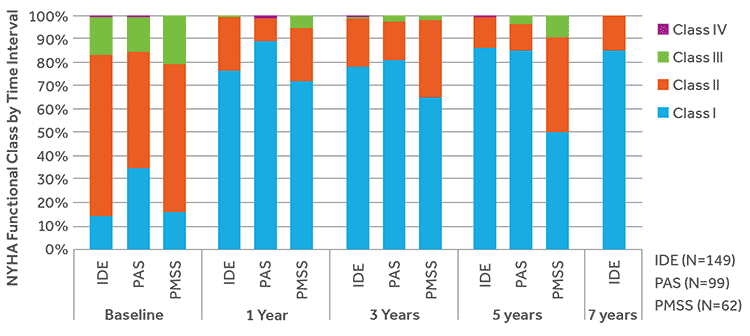
IMPROVES FUNCTIONAL STATUS
At baseline, the majority of subjects in all 3 studies were NYHA class II/III. Following Melody TPV implant, the majority of subjects were in NYHA class I, which remained consistent during follow-up.
Approved for Use in Dysfunctional Surgical Bioprosthetic Pulmonary Valves
Data pooled from two US prospective studies that included both failed conduits and BPVs and one retrospective study assessing Melody in dysfunctional BPVs only, demonstrated safety and effectiveness in restoring pulmonary valve function without open heart surgery.
The following outcomes demonstrate the safety and effectiveness of the Melody Transcatheter Pulmonary Valve (TPV) implanted in a bioprosthetic pulmonary valve (BPV) restoring pulmonary valve competency while delaying the need for surgical intervention.
| Variable | Bioprosthesis (n=125) | RVOT Conduit (n=225) | ||
|---|---|---|---|---|
| Number of Subjects in the Analysis |
Endpoint Rate (95% CI) |
Number of Subjects in the Analysis |
Endpoint Rate (95% CI) |
|
| Procedural success |
117 | 88.9% (82.9%, 93.3%) |
225 | 93.8% (90.4%, 96.2%) |
| Procedurerelated serious AE at 1 year |
125 | 4.0% (2.6%, 10.1%) |
225 | 12.4% (12.0%, 20.0%) |
| Device related serious AE at 1 year |
125 | 2.4% (0.6%, 6.0%) |
225 | 16.0% (16.7%, 25.6%) |
The confidence intervals are exact (Clopper-Pearson) confidence intervals for the binomial proportion.
| Variable | Bioprosthesis (n=125) | RVOT Conduit (n=225) | ||
|---|---|---|---|---|
| Number of Subjects in the Analysis |
1-year Freedom Rate (95% CI) |
Number of Subjects in the Analysis |
1-year Freedom Rate (95% CI) |
|
| TPV Dysfunction |
125 | 97.4% (90.0%, 99.4%) |
223 | 94.1% (90.1%, 96.6%) |
| Reoperation | 125 | 100.0% (NA) |
223 | 98.6% (95.9%, 99.6%) |
| Reintervention | 125 | 100.0% (NA) |
223 | 98.2% (95.2%, 99.3%) |
| All-Cause Mortality |
125 | 100.0% (NA) |
223 | 99.6% (96.8%, 99.9%) |
| Major Stent Fracture |
125 | 100.0% (NA) |
223 | 97.7% (94.6%, 99.1%) |
| Endocarditis | 125 | 100.0% (NA) |
223 | 97.3% (94.0%, 98.8%) |
The cumulative probability of event free estimate is based on the Kaplan-Meier method.
The 95% confidence interval is the loglog transformed 95% Confidence Interval (CI) using
the Peto standard error.


